
What is Glycosylation
Glycosylation is a reaction in which a carbohydrate is attached to a hydroxyl or other functional group of another molecule.
There are many examples of natural molecules that gain, lose or change properties when carbohydrates are attached. Hydroxylation, methylation, fluorination etc. are part of the everyday tool box for many chemists. Glycosylation is more unusual.
Glycosylation chemistry is difficult. Molecules with glycosylatable functional groups (-OH, -COOH, -NH, -SH) often need to have some of those groups blocked so that the sugar can be put where it is needed.
We take a natural approach.
Glycosyltransferase enzymes can glycosylate almost any molecule that has an appropriate side group. They can often work without any need to block side groups and many will work stereo-specifically.
By selecting the right enzyme we can also add multiple different sugars (glucose, galactose, xylose, glucuronic acid, rhamnose etc.).
We can also optimize (“evolve”) an enzyme to achieve a desired profile.
We’re happy to talk to you to see if glycosylation can work for your project. Get in touch by email info@gly-it.com or use the form on the contact page.
Some ways that Glycosylation has worked:
Improved Solubility – Curcumin solubility improved 230x with one glucose unit added. And 22 million times with six glucose units.
Optimised Drug Delivery – Glucosylated Vitamin E diffuses through the skin and is metabolized to the active form.
Improved BBB Penetration – Glucosylated endorphins are two times better taken up in the brain, with better analgesic effects.
Optimised Tissue Targeting – 60% of oral glucosylated dexamethasone reaches the lower bowel, compared with just 1% of the non-glucosylated form.
Improved Activity – morphine-6-glucuronide is 45x-60x more potent as an analgesic than morphine in animal models. With a longer lasting effect.
Modified organoleptic properties – Rebaudioside M works better as a sweetener than all other rebaudiosides tested.
Created novel molecules – with potentially novel utilities and refreshed Intellectual property.
Improve scalability of production
Di-Glycosylation
Di-glycosylation is the attachment of additional sugar molecules to sugars which are already attached to a small molecule.
These structures are plentiful in nature and include Stevia sweeteners, anthocyanin colorants and saponins. Physicochemical properties and biological activities change with every sugar added. So, with each primary sugar containing multiple glycosylatable hydroxy groups the combinatorial space is almost endless.
For example the Stevia sweetener Rebaudioside M has two primary glucose molecules attached and four secondary glucoses attached to these (see illustration below), with a mixture of 1,2- and 1,3-bonds. Rebaudioside M is a better sweetener than all other rebaudiosides.
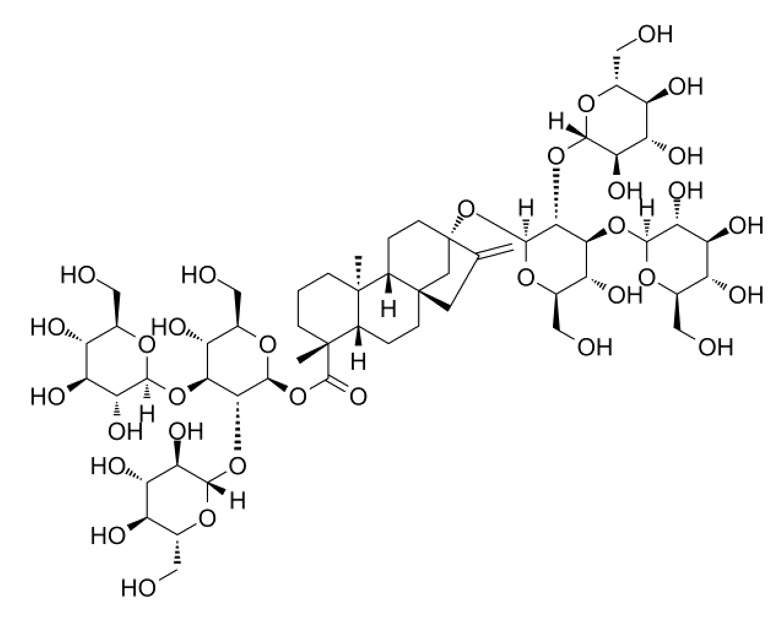
Some UGT enzymes are specifically good at di-glycosylating hydroxy group 2 of the primary sugar, others work on group 3 or group 6, creating 1,2-, 1,3- or 1,6-bonds. GLY-Kit includes examples of all of these enzymes.